 English name; Phthalic anhydride
CAS RN; 85-44-9
EINECS number; 201-607-5
Molecular formula; C8H4O3
Molecular weight; one hundred and forty-eight point one two
Physical and chemical properties; Appearance: White needle shaped crystals with a slight odor.
Melting point; 131.6℃
Boiling point; 295℃
Relative density; one point five two seven
Flash point; 151.7℃
Structural formula: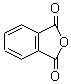
Solubility; Slightly soluble in hot water and ether, soluble in ethanol, benzene, and pyridine.
Purpose; Used as an analytical reagent and also for organic synthesis
Production process of phthalic anhydride
1. In the presence of vanadium pentoxide catalyst, naphthalene and air undergo oxidation reaction in a fluidized bed reactor, followed by condensation, vacuum distillation, and separation;
2. O-xylene is oxidized with air in the presence of vanadium pentoxide catalyst and then refined.
Introduction to phthalic anhydride
Phthalic anhydride, also known as phthalic anhydride, is abbreviated as PA. It is a white flake like solid or powder, or white needle shaped crystals, with a slight odor, a specific gravity of 1.527 (4 ℃), a melting point of 130.8 ℃, a boiling point of 284.5 ℃, easy to sublime, slightly soluble in cold water, easily soluble in hot water and hydrolyzed to phthalic acid, soluble in ethanol, benzene and pyridine, and slightly soluble in ether.
Pure substances are white needle shaped crystals or white flake like solids, easily sublimated, and have water absorption properties; Slightly soluble in cold water and ether, soluble in ethanol, benzene, chloroform, easily soluble in hot water and hydrolyzed to phthalic acid, flammable, and has a risk of combustion when exposed to open flames and strong oxidants. Density 1.527 g/cm3, flash point 152 ℃ (open cup), self ignition point 580 ℃. Toxic, irritating to eyes, respiratory system, and skin. The maximum allowable concentration in the air is 2 PPM.
|
